Standardized Centella asiatica extract ECa 233 alleviates pain hypersensitivity by modulating P2X3 in trigeminal neuropathic pain
DOI:
https://doi.org/10.1590/1678-7757-2023-0337%20Keywords:
ECa 233, Infraorbital nerve chronic constriction, P2X3, NaV1, c-fosAbstract
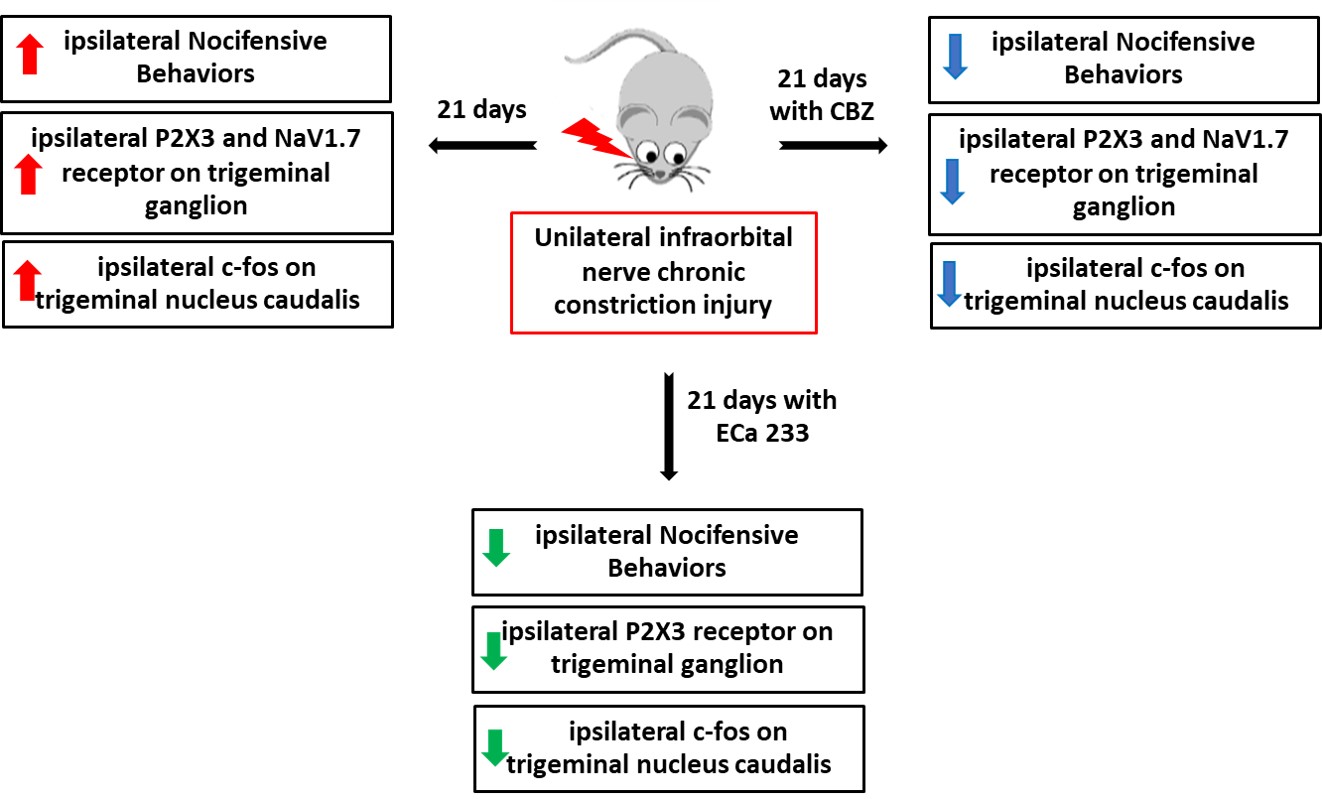
During oral surgery and temporomandibular joint repositioning, pain hypersensitivity often occurs due to irritation or inflammation of the nerve endings in the orofacial region. Objective: This study aimed to investigate the effects of ECa 233, a Centella asiatica–standardized extract, on the development of mechanical hyperalgesia and allodynia induced by chronic constriction injury of the infraorbital nerve in mice. Methodology: The right infraorbital nerves of the mice were ligated. Oral carbamazepine (20 mg/kg) or ECa 233 (30, 100, or 300 mg/kg) was administered daily for 21 days. Von Frey and air-puff tests were performed on both sides of the whisker pad on days 0, 7, 14, and 21. Thereafter, the expression of purinergic receptor subtype 3 (P2X3) and voltage-gated sodium channel 1.7 (NaV1.7), a transmembrane protein, in the trigeminal ganglion and c-fos immunoreactivity-positive neurons in the trigeminal nucleus caudalis was assessed. Results: After 21 days of infraorbital nerve ligation, the mice showed allodynia- and hyperalgesia-like behavior, P2X3 and NaV1.7 were upregulated in the trigeminal ganglion, and nociceptive activity increased in the trigeminal nucleus caudalis. However, the oral administration of carbamazepine (20 mg/kg), ECa 233 (100 mg/kg), or ECa 233 (300 mg/kg) mitigated these effects. Nevertheless, ECa 233 failed to affect NaV1.7 protein expression. Conclusion: Carbamazepine and ECa 233 can prevent pain hypersensitivity in mice. Considering the side effects of the long-term use of carbamazepine, ECa 233 monotherapy or combined ECa 233 and carbamazepine therapy can be used as an alternative for regulating the development of hypersensitivity in trigeminal pain. However, further detailed clinical studies should be conducted to provide comprehensive information on the use of ECa 233.
Downloads
References
Zakrzewska JM, Linskey ME. Trigeminal neuralgia. BMJ. 2014;348:g474. doi: 10.1136/bmj.g474
Sessle BJ. Chronic orofacial pain: Models, mechanisms, and genetic and related environmental influences. Int J Mol Sci. 2021;22(13):7112. doi: 10.3390/ijms22137112
Qi D, Yang Y, Ji P, Kong J, Wu Q, Si H. Upregulation of the purinergic receptor subtype P2X3 in the trigeminal ganglion is involved in orofacial pain induced by occlusal interference in rats. J Oral Facial Pain Headache. 2016;30(1):51-60. doi: 10.11607/ofph.1459
Hsieh YL, Chiang H, Lue JH, Hsieh ST. P2X3-mediated peripheral sensitization of neuropathic pain in resiniferatoxin-induced neuropathy. Exp Neurol. 2012;235(1):316-25. doi: 10.1016/j.expneurol.2012.02.013
Wanasuntronwong A, Punyawattananon V, Rotpenpian N, Meepong R, Srikiatkhachorn A. Nociceptive receptors are expressed differently in trigeminal nociception after lingual nerve injury and unilateral external carotid artery occlusion in rats. Arch Oral Biol. 2021;126:105128. doi: 10.1016/j.archoralbio.2021.105128
Xu W, Zhang J, Wang Y, Wang L, Wang X. Changes in the expression ofvoltage-gated sodium channels Nav1.3, Nav1.7, Nav1.8, and Nav1.9 in rat trigeminal ganglia following chronic constriction injury. Neuroreport. 2016;27(12):929-34. doi: 10.1097/WNR.0000000000000632
Liu BW, Zhang J, Hong YS, Li NB, Liu Y, Zhang M, et al. NGF-Induced Nav1.7 upregulation contributes to chronic post-surgical pain by
activating SGK1-dependent Nedd4-2 phosphorylation. Mol Neurobiol. 2021;58(3):964-82. doi: 10.1007/s12035-020-02156-1
Spina A, Mortini P, Alemanno F, Houdayer E, Iannaccone S. Trigeminal neuralgia: toward a multimodal approach. World Neurosurg. 2017;103:220-30. doi: 10.1016/j.wneu.2017.03.126
Al-Quliti KW. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. Neurosciences
(Riyadh). 2015;20(2):107-14. doi: 10.17712/nsj.2015.2.20140501
Avinash A, Amberkar VM, Kunder SK, Madhyastha S, Meenakumari K. Carbamazepine-induced life-threatening Stevens-Johnson
syndrome and agranulocytosis: The maiden case. J Clin Diagn Res. 2016;10(12):FD01-3. doi: 10.7860%2FJCDR%2F2016%2F23748.9065
Wanasuntronwong A, Tantisira MH, Tantisira B, Watanabe H. Anxiolytic effects of standardized extract of Centella asiatica (ECa
after chronic immobilization stress in mice. J Ethnopharmacol. 2012;143(2):579-85. doi: 10.1016/j.jep.2012.07.010
Chivapat S, Tantisira M. Acute and sub-chronic toxicity of a standardized extract of Centella asiatica ECa 233. Thai J Pharm Sci. 2011;35:55-64.
Buapratoom A, Wanasuntronwong A, Khongsombat O, Tantisira, MH. Anti-nociceptive effects of ECa 233 a standardized extract of
Centella asiatica (L.) urban on chronic neuropathic orofacial pain in mice. J Ethnopharmacol. 2022;283:114737. doi: 10.1016/j.jep.2021.114737
Rotpenpian N, Arayapisit T, Roumwong A, Pakaprot N, Tantisira M, Wanasuntronwong A. A standardized extract of Centella asiatica (ECa
prevents temporomandibular joint osteoarthritis by modulating the expression of local inflammatory mediators in mice. J Appl Oral Sci. 2021;29:e20210329. doi: 10.1590/1678-7757-2021-0329
Li H, Gong X, Zhang L, Zhang Z, Luo F, Zhou Q, et al. Madecassoside attenuates inflammatory response on collagen-induced arthritis in
DBA/1 mice. Phytomedicine. 2009;16(6-7):538-46. doi: 10.1016/j.phymed.2008.11.002
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109-10.
doi: 10.1016/0304-3959(83)90201-4
Martin YB, Avendano C. Effects of removal of dietary polyunsaturated fatty acids on plasma extravasation and mechanical allodynia in
a trigeminal neuropathic pain model. Mol Pain. 2009;5:8-17. doi: 10.1186/1744-8069-5-8
Rotpenpian N, Tapechum S, Vattarakorn A, Chindasri W, Care C, Pakaprot N, et al. Evolution of mirror-image pain in temporomandibular
joint osteoarthritis mouse model. J Appl Oral Sci. 2021;29:e20200575. doi: 10.1590/1678-7757-2020-0575
Krzyzanowska A, Pittolo S, Cabrerizo M, Sánchez-López J, Krishnasamy S, Venero C, et al. Assessing nociceptive sensitivity
in mouse models of inflammatory and neuropathic trigeminal pain. J Neurosci Methods. 2011;201(1):46-54. doi: 10.1016/j.
neumeth.2011.07.006
Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General pathways of pain sensation and the major neurotransmitters
involved in pain regulation. Int J Mol Sci. 2018;19(8):2164. doi: 10.3390/ijms19082164
Seifert O, Baerwald C. Interaction of pain and chronic inflammation. Z Rheumatol. 2021;80(3):205-13. doi: 10.1007/s00393-020-00951-8
Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. Pain. 2009;141(1-2):127-34. doi: 10.1016/j.pain.2008.10.024
Hsieh YL, Chiang H, Lue JH, Hsieh ST. P2X3-mediated peripheral sensitization of neuropathic pain in resiniferatoxin-induced
neuropathy. Exp Neurol. 2012;235(1):316-25. doi: 10.1016/j.expneurol.2012.02.013
Koizumi M, Asano S, Furukawa A, Hayashi Y, Hitomi S, Shibuta I, et al. P2X3 receptor upregulation in trigeminal ganglion neurons through
TNFα production in macrophages contributes to trigeminal neuropathic pain in rats. J Headache Pain. 2021;22(1):31. doi: 10.1186/s10194-021-01244-4
Lee JT, Shanina I, Chu YN, Horwitz MS, Johnson JD. Carbamazepine, a beta-cell protecting drug, reduces type 1 diabetes incidence in NOD
mice. Sci Rep. 2018;8(1):4588. doi: 10.1038/s41598-018-23026-w
Sukketsiri W, Tanasawet S, Moolsap F, Tantisira MH, Hutamekalin P, Tipmanee V. ECa 233 suppresses LPS-induced proinflammatory
responses in macrophages via suppressing ERK1/2, p38 MAPK and Akt pathways. Biol Pharm Bull. 2019;42(8):1358-65. doi: 10.1248/bpb.b19-00248
Fredriksson L, Wink S, Herpers B, Benedetti G, Hadi M, Bont H, et al. Drug-induced endoplasmic reticulum and oxidative stress responses independently sensitize toward TNFα-mediated hepatotoxicity. Toxicol Sci. 2014;140(1):144-59. doi: 10.1093/toxsci/kfu072
Li N, Liu B, Wu W, Hong Y, Zhang J, Liu Y, et al. Upregulation of transcription factor 4 downregulates NaV1.8 expression in DRG neurons and prevents the development of rat inflammatory and neuropathic hypersensitivity. Exp Neurol. 2020;327:113240. doi: 10.1016/j.expneurol.2020.113240
Yang B, Xu Y, Hu Y, Luo Y, Lu X, Tsui CK, et al. Madecassic acid protects against hypoxia-induced oxidative stress in retinal
microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed Pharmacother. 2016;84:845-52. doi: 10.1016/j.biopha.2016.10.015
Chung L. A brief introduction to the transduction of neural activity into Fos signal. Dev Reprod. 2015;19(2):61-7. doi: 10.12717/DR.2015.19.2.061
Songvut P, Chariyavilaskul P, Tantisira M, Khemawoot P. Safety and pharmacokinetics of standardized extract of Centella asiatica (ECa 233) capsules in healthy Thai volunteers: a phase 1 clinical study. Planta Med. 2019;85(6):483-90. doi: 10.1055/a-0835-6671
Downloads
Published
Versions
- 2024-03-25 (4)
- 2024-02-07 (3)
- 2024-02-07 (2)
- 2024-02-07 (1)
Issue
Section
License
Copyright (c) 2024 Journal of Applied Oral Science

This work is licensed under a Creative Commons Attribution 4.0 International License.
Todo o conteúdo do periódico, exceto onde está identificado, está licenciado sob uma Licença Creative Commons do tipo atribuição CC-BY.

