Lipid nanocarrier containing eugenol for denture hygiene: evaluation of efficacy against Candida biofilms
DOI:
https://doi.org/10.1590/1678-7757-2024-0455Keywords:
Eugenol, Nanotechnology, Biofilms, Candida, Acrylic resinsAbstract
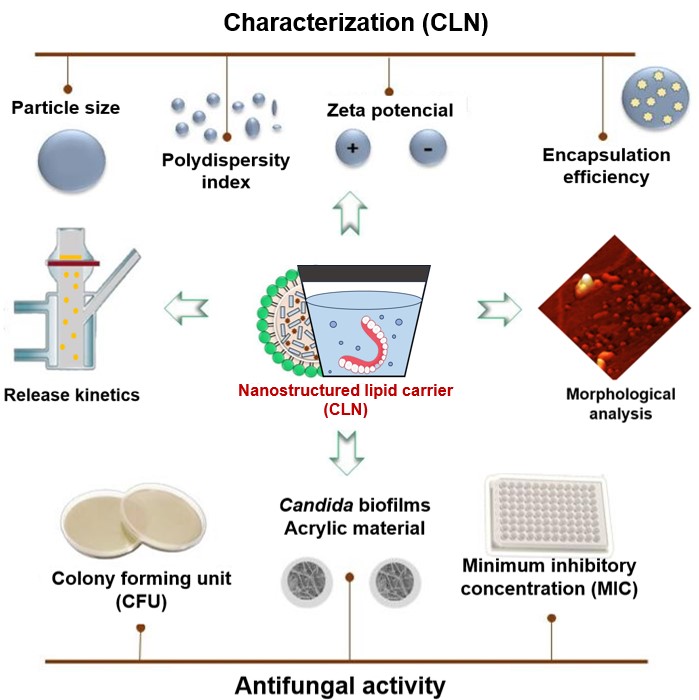
Eugenol has demonstrated efficacy against Candida spp., which is highly prevalent in denture wearers. However, the low water solubility and high volatility limit its application. The encapsulation in nanostructured lipid carriers (NLCs) may be a viable approach for developing new sanitizing agents for denture hygiene. Objective To develop a sanitizing dispersion for denture hygiene using nanostructured lipid carriers (NLCs) containing eugenol and to evaluate the efficacy against Candida spp. biofilms. Methodology The formulation was prepared using the ultrasonication method and characterized in terms of particle size (PS), polydispersity index (PDI), zeta potential (ZP), and encapsulation efficiency (EE). The minimum inhibitory concentration (MIC) was determined by the broth microdilution method and the antifungal activity was evaluated by four treatment groups (nanostructured formulation containing eugenol (NFE), free eugenol (FE), saline solution (SS), and the drug-free formulation NFW after eight hours of immersion in biofilms of two Candida species (Candida albicans and Candida glabrata) adhered to polymethyl methacrylate resin specimens. Results The nanoparticles of NFE showed a particle size of 199.5±2.55 nanometers (nm) as measured by DLS, high homogeneity (0.07±0.02), an EE of 83.07±0.23, and a negative ZP (-25.86±0.65). The MICs of FE for Candida albicans and Candida glabrata were up to 10 times (64 µg/mL) and eight times (128 µg/mL) higher, respectively, than the MICs of NFE (6 µg/mL and 16 µg/mL). The biofilms of these microorganisms showed a significant reduction after immersion in NFE compared to the other tested groups (FE, NBF, and SS) (P<0.0001). Conclusion The NFE demonstrated fungicidal activity against the isolated strains and significantly reduced Candida biofilms, thus showing promising performance for the sanitization of dentures over eight hours.
Downloads
References
Rocha R, Santos G, Duarte TN, Corrêa GO, Nampo FK, Ramos SP, et al. Chemical cleaning methods for prostheses colonized by Candida spp: a systematic review. J Prosthet Dent. 2020;124(6):653-58. doi: 10.1016/j.prosdent.2019.10.004. »https://doi.org/10.1016/j.prosdent.2019.10.004.
Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253-73. doi: 10.1128/CMR.00076-09.
»https://doi.org/10.1128/CMR.00076-09.
El-Baz AM, Mosbah RA, Goda RM, Mansour B, Sultana T, Dahms TE, et al. Back to nature: Combating candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics (Basel). 2021;10(1):1-18. doi: 10.3390/antibiotics10010001.
»https://doi.org/10.3390/antibiotics10010001.
Kilic K, Koc AN, Tekinsen FF, Yildiz P, Kilic D, Zararsiz G, et al. Assessment of Candida species colonization and denture-related stomatitis in bar- and locator-retained overdentures. J Oral Implantol. 2014;40(5):549-56. doi: 10.1563/AAID-JOI-D-12-00048. »https://doi.org/10.1563/AAID-JOI-D-12-00048.
Armstrong-James D, Brown GD, Netea MG, Zelante T, Gresnigt MS, van de Veerdonk FL, et al. Immunotherapeutic approaches to treatment of fungal diseases. Lancet Infect Dis. 2017;17(12):393-402. doi: 10.1016/S1473-3099(17)30442-5. »https://doi.org/10.1016/S1473-3099(17)30442-5.
Pereira R, Dos Santos Fontenelle RO, de Brito EH, de Morais SM. Biofilm of Candida albicans: formation, regulation and resistance. J Appl Microbiol. 2021;131(1):11-22. doi: 10.1111/jam.14949. »https://doi.org/10.1111/jam.14949.
Schmutzler A, Rauch A, Nitschke I, Lethaus B, Hahnel S. Cleaning of removable dental prostheses:a systematic review. J Evid Based Dent Pract. 2021;21(4):101644. doi: 10.1016/j.jebdp.2021.101644. »https://doi.org/10.1016/j.jebdp.2021.101644.
Papadopoulos T, Polyzois G, Tapanli A, Frangou M. The effect of disinfecting solutions on bending properties and weight changes of Co-Cr and Ti-6Al-7Nb alloys for dentures. Odontology. 2011;99(1):77-82. doi: 10.1007/s10266-010-0135-2. »https://doi.org/10.1007/s10266-010-0135-2.
Slaughter RJ, Watts M, Vale JA, Grieve JR, Schep LJ. The clinical toxicology of sodium hypochlorite. Clin Toxicol (Phila). 2019;57(5):303-311. doi: 10.1080/15563650.2018.1543889. »https://doi.org/10.1080/15563650.2018.1543889.
Paranhos HF, Bezzon OL, Davi LR, Felipucci DN, Silva CH, Pagnano VO. Effect of cleanser solutions on the color of acrylic resins associated with titanium and nickel-chromium alloys. Braz Oral Res. 2014;28:0017. doi: 10.1590/1807-3107bor-2014.vol28.0017. »https://doi.org/10.1590/1807-3107bor-2014.vol28.0017.
Arruda CN, Salles MM, Oliveira VC, Macedo AP, da Silva CH, Paranhos HF. Using denture cleansers to control biofilm from dentures and brushes: a randomized crossover clinical trial. Int J Prosthodont. 2021;34(3):291-299. doi: 10.11607/ijp.6665. »https://doi.org/10.11607/ijp.6665.
Saracino IM, Foschi C, Pavoni M, Spigarelli R, Valerii MC, Spisni E. Antifungal activity of natural compounds vs. candida spp.: a mixture of cinnamaldehyde and eugenol shows promising in vitro results. Antibiotics (Basel). 2022;11(1):73. doi: 10.3390/antibiotics11010073.
»https://doi.org/10.3390/antibiotics11010073.
Martins C, Doran C, Laires A, Rueff J, Rodrigues AS. Genotoxic and apoptotic activities of the food flavourings myristicin and eugenol in AA8 and XRCC1 deficient EM9 cells. Food Chem Toxicol. 2011;49(2):385-92. doi: 10.1016/j.fct.2010.11.013. »https://doi.org/10.1016/j.fct.2010.11.013.
Fuentes C, Fuentes A, Barat JM, Ruiz MJ. Relevant essential oil components: a minireview on increasing applications and potential toxicity. Toxicol Mech Methods. 2021;31(8):559-565. doi: 10.1080/15376516.2021.1940408. »https://doi.org/10.1080/15376516.2021.1940408.
Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs: a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113(1-3):151-70. doi: 10.1016/j.jbiotec.2004.06.007. »https://doi.org/10.1016/j.jbiotec.2004.06.007.
Souto EB, Müller RH, Gohla S. A novel approach based on lipid nanoparticles (SLN) for topical delivery of alpha-lipoic acid. J Microencapsul. 2005;22(6):581-92. doi: 10.1080/02652040500162378. »https://doi.org/10.1080/02652040500162378.
Garg A, Singh S. Enhancement in antifungal activity of eugenol in immunosuppressed rats through lipid nanocarriers. Colloids Surf B Biointerfaces. 2011;87(2):280-8. doi: 10.1016/j.colsurfb.2011.05.030. »https://doi.org/10.1016/j.colsurfb.2011.05.030.
Elmowafy M, Al-Sanea MM. Nanostructured lipid carriers (NLCs) as drug delivery platform: advances in formulation and delivery strategies. Saudi Pharm J. 2021;29(9):999-1012. doi: 10.1016/j.jsps.2021.07.015. »https://doi.org/10.1016/j.jsps.2021.07.015.
Vijayakumar A, Baskaran R, Jang YS, Oh SH, Yoo BK. Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. aaps pharmscitech. 2017;18(3):875-883. doi: 10.1208/s12249-016-0573-4. »https://doi.org/10.1208/s12249-016-0573-4.
Lopes CE, Langoski G, Klein T, Ferrari PC, Farago PV. A simple HPLC method for the determination of halcinonide in lipid nanoparticles: development, validation, encapsulation efficiency, and in vitro drug permeation. Braz J Pharm Sci. 2017;53(2):e15250. doi: 10.1590/s2175-97902017000215250. »https://doi.org/10.1590/s2175-97902017000215250.
Eaton P, Quaresma P, Soares C, Neves C, Almeida MP, Pereira E, et al. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy. 2017;182:179-90. doi: 10.1016/j.ultramic.2017.07.001. »https://doi.org/10.1016/j.ultramic.2017.07.001.
Monton C, Settharaksa S, Suksaereeb J, Chusuta T. The preparation, characterization, and stability evaluation of a microemulsion-based oral spray containing clove oil for the treatment of oral candidiasis. J Drug Deliv Sci Technol. 2020;57:101735. doi: 10.1016/j.jddst.2020.101735. »https://doi.org/10.1016/j.jddst.2020.101735.
Leal AL, Bezerra CF, Rocha JE, Santos AT, Cruz RP, Carneiro JN, et al. Piper cernuum Vell.: Chemical profile and antimicrobial potential evaluation. Ind Crops Prod. 2019;140:111577. doi: 10.1016/j.indcrop.2019.111577. »https://doi.org/10.1016/j.indcrop.2019.111577.
Fang Z, Bhandari B. Encapsulation of polyphenols: a review. Trends Food Sci Technol. 2010;21:510-23. doi: 10.1016/j.tifs.2010.08.003.
»https://doi.org/10.1016/j.tifs.2010.08.003.
Silva AC, González-Mira E, García ML, Egea MA, Fonseca J, Silva R, et al. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): high pressure homogenization versus ultrasound. Colloids Surf B Biointerfaces. 2011;86(1):158-65. doi: 10.1016/j.colsurfb.2011.03.035. »https://doi.org/10.1016/j.colsurfb.2011.03.035.
Beloqui A, Solinís MÁ, Rodríguez-Gascón A, Almeida AJ, Préat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12(1):143-61. doi: 10.1016/j.nano.2015.09.004. »https://doi.org/10.1016/j.nano.2015.09.004.
Müller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr Drug Discov Technol. 2011;8(3):207-27. doi: 10.2174/157016311796799062. »https://doi.org/10.2174/157016311796799062.
Santos SV, Badan RA, Andrade SM. Solid lipid nanoparticles as carriers for lipophilic compounds for applications in foods. Food Res Int. 2019;122:610-626. doi: 10.1016/j.foodres.2019.01.032. »https://doi.org/10.1016/j.foodres.2019.01.032.
Robles LV, García FB, Garzón SM, Hernández LA, Vázquez RM. Nanopartículas lipídicas sólidas [Solid lipid nanoparticles]. Rev Mex Cienc Farm. 2008;39(1):38-52. Spanish.
Yoon G, Park JW, Yonn I. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. J Pharm Investig. 2013;43:353-362. doi: 10.1007/s40005-013-0087-y. »https://doi.org/10.1007/s40005-013-0087-y.
Domingo C, Saurina J. An overview of the analytical characterization of nanostructured drug delivery systems: towards green and sustainable pharmaceuticals: a review. Anal Chim Acta. 2012;744:8-22. doi: 10.1016/j.aca.2012.07.010. »https://doi.org/10.1016/j.aca.2012.07.010.
Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol. 2013;19:29-43. doi: 10.1016/j.ifset.2013.03.002. »https://doi.org/10.1016/j.ifset.2013.03.002.
Bera B. Nanoporous silicon prepared by vapour phase strain etch and sacrificial technique. In: Proceedings of the International Conference on Microelectronic Circuit and System (Micro) [Internet]. 2015 [cited 2025 Jan 20];1:42-45. Available from: https://www.ijcaonline.org/proceedings/micro2015/number1/23705-1742/ »https://www.ijcaonline.org/proceedings/micro2015/number1/23705-1742/
Radtke M, Souto EB, Müller RH. Nanostructured lipid carriers: a novel generation of solid lipid drug carriers. Pharm Technol Eur. 2005;17(4):45-50.
Krambeck K, Silva V, Silva R, Fernandes C, Cagide F, Borges F, et al. Design and characterization of Nanostructured lipid carriers (NLC) and Nanostructured lipid carrier-based hydrogels containing Passiflora edulis seeds oil. Int J Pharm. 2021;600:120444. doi: 10.1016/j.ijpharm.2021.120444. »https://doi.org/10.1016/j.ijpharm.2021.120444.
Redhead HM, Davis SS, Illum L. Drug delivery in poly(lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: in vitro characterisation and in vivo evaluation. J Control Release. 2001;70(3):353-63. doi: 10.1016/s0168-3659(00)00367-9
»https://doi.org/10.1016/s0168-3659(00)00367-9.
Cheow WS, Hadinoto K. Factors affecting drug encapsulation and stability of lipid-polymer hybrid nanoparticles. Colloids Surf B Biointerfaces. 2011;85(2):214-220. doi: 10.1016/j.colsurfb.2011.02.033. »https://doi.org/10.1016/j.colsurfb.2011.02.033.
Müller RH, Runge SA, Ravelli V, Thünemann AF, Mehnert W, Souto EB. Cyclosporine-loaded solid lipid nanoparticles (SLN®): Drug-lipid physicochemical interactions and characterization of drug incorporation. Eur J Pharm Biopharm. 2008;68(3):535-44. doi: 10.1016/j.ejpb.2007.07.006. »https://doi.org/10.1016/j.ejpb.2007.07.006.
Espinosa-Olivares MA, Delgado-Buenrostro NL, Chirino YI, Trejo-Márquez MA, Pascual-Bustamante S, Ganem-Rondero A. Nanostructured lipid carriers loaded with curcuminoids: Physicochemical characterization, in vitro release, ex vivo skin penetration, stability and antioxidant activity. Eur J Pharm Sci. 2020;155:105533. doi: 10.1016/j.ejps.2020.105533. »https://doi.org/10.1016/j.ejps.2020.105533.
Friedrich RB, Kann B, Coradini K, Offerhaus HL, Beck RC, Windbergs M. Skin penetration behavior of lipid-core nanocapsules for simultaneous delivery of resveratrol and curcumin. Eur J Pharm Sci. 2015;78:204-13. doi: 10.1016/j.ejps.2015.07.018. »https://doi.org/10.1016/j.ejps.2015.07.018.
He M, Du M, Fan M, Bian Z. In vitro activity of eugenol against Candida albicans biofilms. Mycopathologia. 2007;163(3):137-43. doi: 10.1007/s11046-007-0097-2. »https://doi.org/10.1007/s11046-007-0097-2.
Marcos-Arias C, Eraso E, Madariaga L, Quindós G. In vitro activities of natural products against oral Candida isolates from denture wearers. BMC Complement Altern Med. 2011;11:119. doi: 10.1186/1472-6882-11-119. »https://doi.org/10.1186/1472-6882-11-119.
Ahmad A, Wani MY, Khan A, Manzoor N, Molepo J. Synergistic Interactions of Eugenol-tosylate and Its Congeners with Fluconazole against Candida albicans. PLoS One. 2015;10(12):e0145053. doi: 10.1371/journal.pone.0145053. »https://doi.org/10.1371/journal.pone.0145053.
Lone SA, Ahmad A. Inhibitory effect of novel eugenol tosylate congeners on pathogenicity of Candida albicans. BMC Complement Med Ther. 2020;20:131. doi: 10.1186/s12906-020-02929-0. »https://doi.org/10.1186/s12906-020-02929-0.
Didehdar M, Chegini Z, Shariati A. Eugenol: a novel therapeutic agent for the inhibition of Candida species infection. Front Pharmacol. 2022;13:872127. doi: 10.3389/fphar.2022.872127. »https://doi.org/10.3389/fphar.2022.872127.
Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288-303. doi: 10.4103/1735-5362.235156. »https://doi.org/10.4103/1735-5362.235156.
Akel H, Ismail R, Katona G, Sabir F, Ambrus R, Csóka I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: formulation, characterization, and in vitro evaluation. Int J Pharm. 2021;604:120724. doi: 10.1016/j.ijpharm.2021.120724.
»https://doi.org/10.1016/j.ijpharm.2021.120724.
Viegas C, Patrício AB, Prata JM, Nadhman A, Chintamaneni PK, Fonte P. Solid lipid nanoparticles vs. nanostructured lipid carriers: a comparative Review. Pharmaceutics. 2023;15(6):1593. doi: 10.3390/pharmaceutics15061593. »https://doi.org/10.3390/pharmaceutics15061593.
Nett J, Andes D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol. 2006;9(4):340-5. doi: 10.1016/j.mib.2006.06.007. »https://doi.org/10.1016/j.mib.2006.06.007.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Irisvaldo Lima Guedes, Matheus Oliveira do Nascimento , Leandro de Sousa Dias, Alyne Rodrigues de Araujo-Nobre, Humberto Medeiros Barreto, Érika de Araújo Abi-chacra, Ana Cristina Vasconcelos Fialho, Gláuber Campos Vale, André Luis Menezes Carvalho

This work is licensed under a Creative Commons Attribution 4.0 International License.
Todo o conteúdo do periódico, exceto onde está identificado, está licenciado sob uma Licença Creative Commons do tipo atribuição CC-BY.

